The U.S. Food and Drug Administration (FDA) granted emergency use authorization (EUA) of therapies utilizing monoclonal antibody drugs for the treatment of mild to moderate COVID-19 in adults and pediatric patients with positive results of direct SARS-CoV-2 testing. In this blog, we’ll take a deeper look at monoclonal antibodies along with antiviral agents such as remdesivir that are being used to help combat COVID-19 in patients.
Monoclonal Antibodies
Monoclonal antibodies are lab-synthesized copies of antibodies that are generated by the body’s immune system. Monoclonal antibodies granted EUA include:
- Bamlanivimab
- Bamlanivamab + etesevimab
- Clairivimab + imdevimab (REGEN-COV)
To receive monoclonal antibody therapies, the patient must be:
- 12 years of age or older and weigh at least 40 kg
- At high risk for progressing to severe COVID-19 and/or hospitalization
Patients at high risk include patients exhibiting one or more of the following:
- Body mass index (BMI) > 35
- Chronic kidney disease
- Diabetes
- Immunosuppressive disease
- Currently receiving immunosuppressive treatment
- Age 65 or greater
- Age 55 or greater with any of the following:
- Cardiovascular disease
- Hypertension
- Chronic obstructive pulmonary disease/other chronic respiratory disease
- Age 12–17 and have any of the following:
- BMI > 85th percentile for their age and gender based on CDC growth charts
- Sickle cell disease
- Congenital or acquired heart disease
- Neurodevelopmental disorders such as cerebral palsy
- A medical-related technology dependence such as tracheostomy, gastrostomy, or positive pressure ventilation (not related to COVID-19)
- Asthma, relative airway or other chronic respiratory disease that requires daily medication for control
Monoclonal antibodies are not authorized for use with patients who are hospitalized due to COVID-19, require oxygen therapy due to COVID-19, or require an increase in baseline oxygen flow rate due to COVID-19 in those on chronic oxygen therapy due to underlying non-COVID-19 related comorbidity.
According to the National Institutes of Health (NIH), antibody-based therapies may have their greatest likelihood of having an effect in the earliest stages of infection before the host has mounted an effective immune response. However, there is insufficient data to recommend either for or against the use of antibody therapy in patients with mild to moderate COVID-19 who are not hospitalized.
Antiviral Agents
Remdesivir, an antiviral agent, is approved by the FDA for the treatment of COVID-19 and recommended for use in hospitalized patients who require supplemental oxygen. However, it is not routinely recommended for patients who require mechanical ventilation due to the lack of data showing benefit at this advanced stage of the disease.
Dexamethasone, a corticosteroid, has been found to improve survival in hospitalized patients who require supplemental oxygen, with the greatest effect observed in patients who require mechanical ventilation. Therefore, the use of dexamethasone is strongly recommended in this setting.
National Institutes of Health (NIH) Recommendations
For patients who are hospitalized and require supplemental oxygen, remdesivir is recommended for patients who require "minimal supplemental oxygen," with dexamethasone and remdesivir recommended for patients who require "increasing amounts" of supplemental oxygen. For hospitalized patients who do not require supplemental oxygen, the NIH notes there is currently insufficient data to recommend for or against the use of remdesivir.
The NIH COVID-19 treatment guidelines are captured in the chart below: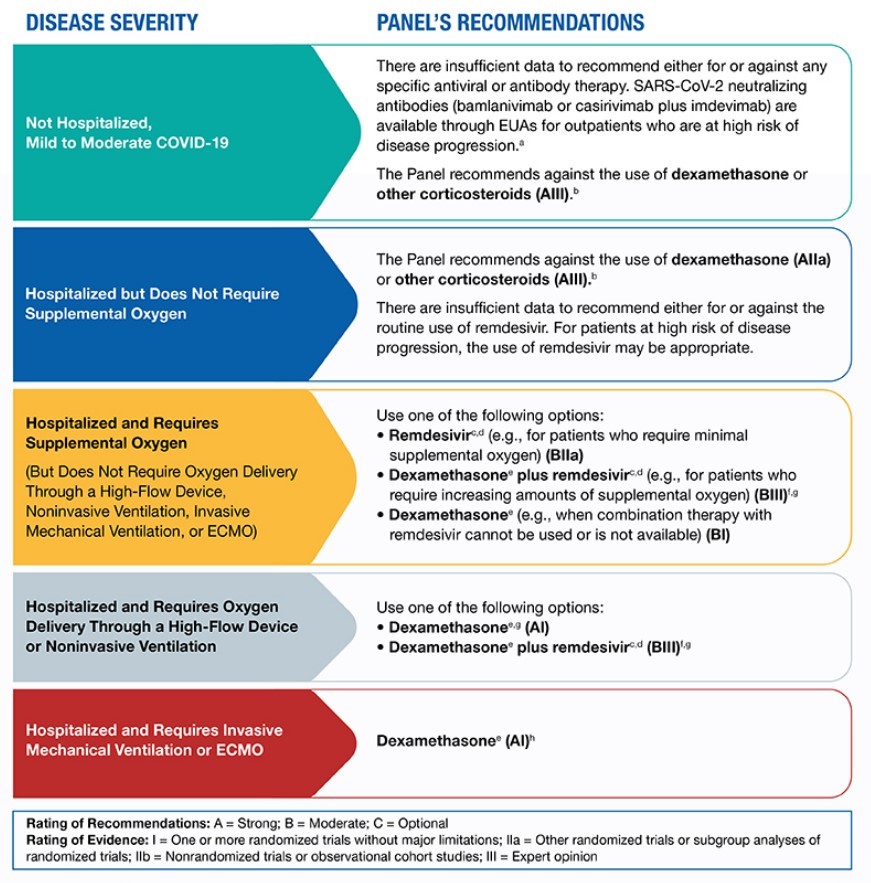 Other potential therapies include:
Other potential therapies include:
- Ivermectin
- Tested in four hospitals in Florida early in the crisis (March 15 – May 11, 2020)
- Lower mortality with ivermectin therapy (15% vs. 25% in patients undergoing standard care)
- Due to significant methodological flaws and incomplete information, the NIH does not recommend for or against ivermectin therapy
- Colchicine
- Final text showed that colchicine did not reduce ventilation or death alone
- Fivefold increase in pulmonary embolism
- Trial stopped early and did not enroll 6,000 patients without consulting DSMB
- Lead investigator did not disclose owned patents for colchicine
Conclusions
For patients who fall into high-risk categories, monoclonal antibodies administered early in disease progression appear to help prevent hospitalizations. For patients already hospitalized with COVID-19, antiviral therapies such as remdesivir and dexamethasone have been used to help those with more severe illness shorten the length of hospitalization. However, the NIH does not recommend remdesivir with dexamethasone as an option for hospitalized COVID-19 patients who require ventilation or extracorporeal membrane oxygenation (ECMO), recommending only dexamethasone instead.
For more information, contact CPS: contactus@cpspharm.com.
-----
i https://www.covid19treatmentguidelines.nih.gov/therapeutic-management/. Accessed 3.01.21.
ii Ibid.
ii Ibid.
iv https://www.medpagetoday.com/infectiousdisease/covid19/90008. Accessed 3.01.21.
v Ibid.